Sysmex TBNK test system
Reagent system for reliable lymphocyte subset analysis - now even simpler
- Reliable analysis of T, B and NK cells and CD4+ and CD8+ T-cell subsets
- Absolute counts directly from the analyser – no counting beads required
- Streamlined workflow – from test request to validation and reporting
- Automated acquisition and analysis, and optional automation of sample preparation
Lymphocyte subset testing
Flow cytometric immunophenotyping to evaluate the percentages and absolute counts of lymphocyte subsets is a well-established test that is performed in a variety of settings. It is primarily used for monitoring CD3+CD4+ T cells in human immunodeficiency virus (HIV)-infected individuals, and also in the evaluation of other forms of immune deficiency and autoimmune diseases [1-4].
Unique features of the Sysmex TBNK test system
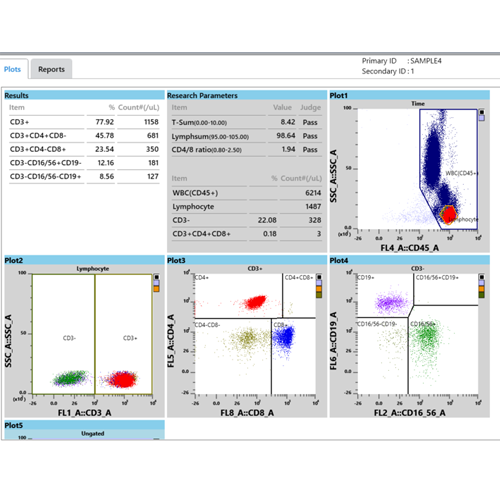
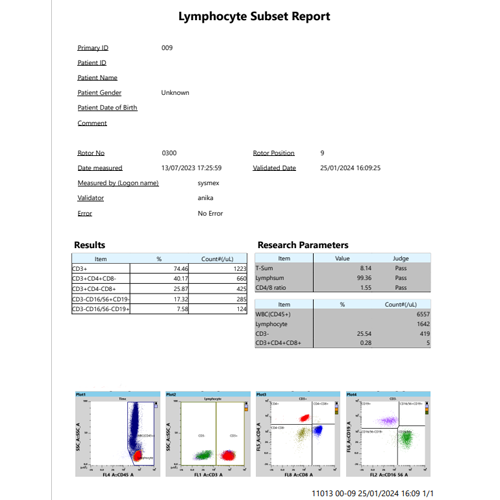
The Sysmex TBNK test system on the XF-1600 platform provides accurate counts directly from the flow cytometer, without the need for counting beads. This is made possible by the volumetric count capability of the XF-1600 based on a calibrated syringe-driven acquisition of exact sample volume for accurate white blood cell (WBC) count – technology that is inherited from Sysmex’s XN-Series haematology analysers.
The resulting absolute counts are metrologically traceable to an internationally recognised reference method for white blood cells (WBC) [5].
Your benefits in routine
- The standardisation and convenience afforded by a preconfigured solution:
- Ready-to-use premixed reagent cocktail
- Easy-to-use XF-1600 TBNK software – with a pre-defined template, automated gating and calculations
- The assurance of reliability through built-in quality control procedures
- Automation options - automated sample acquisition on the XF-1600 flow cytometer and optional automated sample preparation on the PS-10 sample preparation system
- Streamlined workflow – all steps from test request to reporting are streamlined by Sysmex digital solutions
1) Warnatz, K. and Schlesier, M. Flow cytometric phenotyping of common variable immunodeficiency. Cytometry, 2008;74B: 261-271. https://doi.org/10.1002/cyto.b.20432
2) World Health Organisation. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. 2021. ISBN: 978-92-4-003159-3
3) McCusker C, Upton J, Warrington R. Primary immunodeficiency. Allergy Asthma Clin Immunol. 2018;14(Suppl 2):141-152. https://doi.org/10.1186/s13223-018-0290-5
4) Rabson A. Enumeration of T-cells subsets in patients with HIV infection. AIDS Clin Care. 1995 Jan;7(1):1-3. PMID: 11362161.https://pubmed.ncbi.nlm.nih.gov/11362161/
5) Ward RY, Stevens M, Bashir S. Metrological traceability in flow cytometry? Evaluation of a new volumetric method for lymphocyte subsets. Int J Lab Hematol. 2023;1‐7. doi:10.1111/ijlh.1421 https://doi.org/10.1111/ijlh.14219
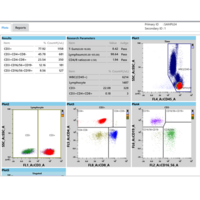
Results
| IVD parameters | Relative percentages** and absolute counts* of:
|
| Research Parameters Analysis results for these research parameters must not be used for diagnosis of patients | Absolute counts* of:
Sums and ratios
|
*Number of cells per μL
**Ratio to lymphocytes (based on a lymphocyte gate)
Sysmex France
Sysmex France
ZAC Paris Nord 2
93420 Villepinte
01 48 17 01 90
TBNK basic online training
After this course you will be able to perform and interpret the TBNK application using the XF-1600. This training is intended for operators to run the TBNK application.
Documents produit
Documents réglementaires
Les documents réglementaires tels que la Notice d’utilisation sont accessibles à l’aide d’un login My Sysmex valide :
Vers My Sysmex